Let’s say your name is Lena. Or Tanya. Or maybe Alex.
It doesn’t matter — what matters is that you’re curious, willing to improve your work with aquatic animals, and finally have access to a microscope (congrats!). You even got some stains? Then let’s begin.
*And please — wear gloves. Not just for your own safety, but for the fish. Their slime coat is thin and fragile after transport, and they don’t need extra stress from human skin contact. *
1. Unpacking the Fish
The fish has spent hours in a plastic bag — **little water, lots of air**, no food before shipment. Waste builds up. The water degrades. Bacteria start to multiply.

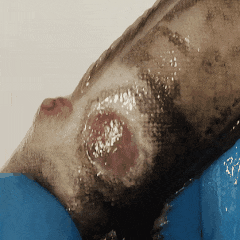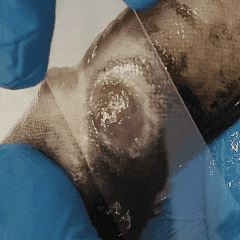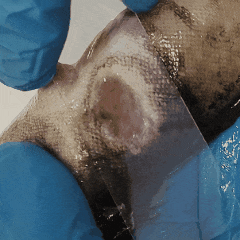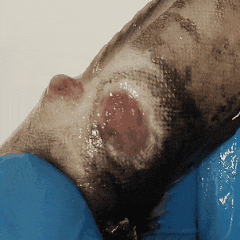
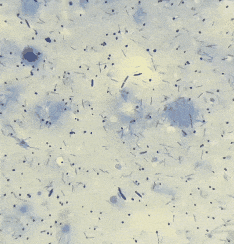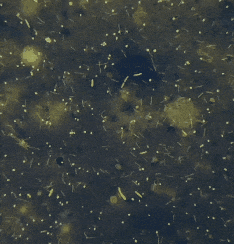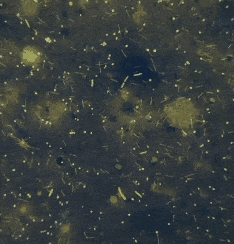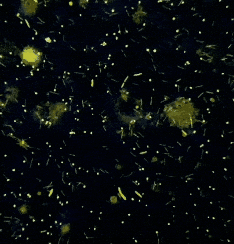
It’s entropy at work. Fish feces are there. Bacterial growth is inevitable.
The best thing you can do? Take skin or wound smears in the first hour after unpacking.
Basic Staining — Löffler’s Methylene Blue
This method gives a quick overview of tissue damage, bacterial presence, fungal elements, etc.
-
Prepare a saturated alcoholic methylene blue solution:
1.6 g in 100 mL of 95% ethanol. -
Mix 30 mL of that with 100 mL of 0.01% KOH solution. (0.01 g KOH in 100 mL distilled water)
-
Final concentrations per 100 mL:
Methylene blue – 0.37 g
Ethanol – 23.08 mL
0.01% KOH – 76.92 mL
Staining time: 10–30 seconds. That’s it.
Polymicrobial Smears
Ways to describe such a smear:
polymicrobial conglomerate
pleomorphic bacterial mass
heterogenous community
mixed biofilm
bacterial debris field
polyculture contamination
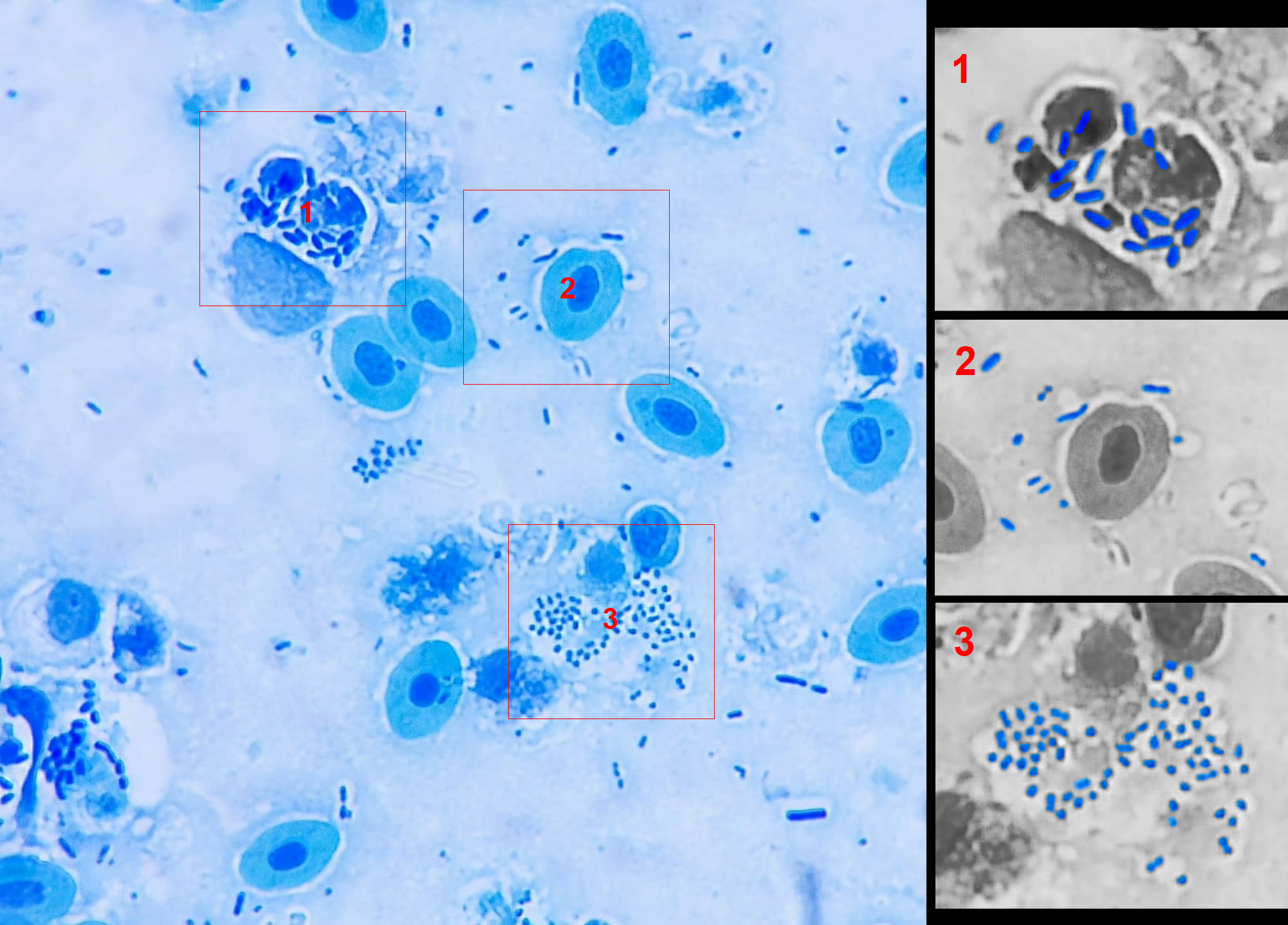
“If the smear looks like bacterial soup — various shapes, sizes, forms — it’s probably just dirt. Not an infection, but a microbial mess caused by bad water, stress, temperature swings. ”
Monomicrobial Smears
Ways to describe such a smear:
monomorphic bacterial colony
homogeneous cluster
pure culture pattern
uniform pathogen presence
mono-infection profile
“If all bacteria look the same — same shape, same size, same reaction — that’s a red flag. Likely a dominant pathogen multiplying aggressively.”
This situation usually requires:
bacterial culture (for ID)
antibiotic susceptibility testing
treatment
What If You See Ulcers?
Ulcers may host mycobacteria, including Mycobacterium marinum (fish TB). These don’t show up with simple stains.
Use Ziehl stain (modified for fish pathology).
Ziehl’s Fuchsin Solution:
- 1 g basic fuchsin in 10 mL 96% ethanol
- Add to 5% phenol solution (100 mL)
- Let it sit 48h and filter
Staining steps:
- Fix smear (air-dry + flame)
- Add filter paper, apply Ziehl stain
- Heat until vapor appears (briefly!)
- Let cool, rinse with water
- Decolorize in 5% sulfuric acid (dip 2–3 times)
- Rinse and counterstain with Löffler’s methylene blue
Result:
Red = acid-fast bacteria (e.g., mycobacteria)
Blue = background tissue & non–acid-fast
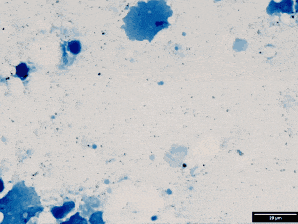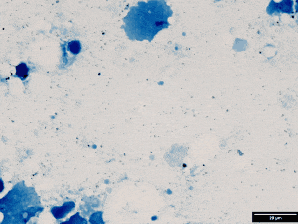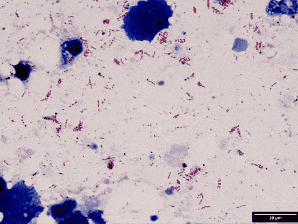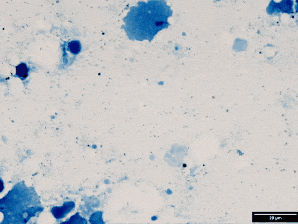
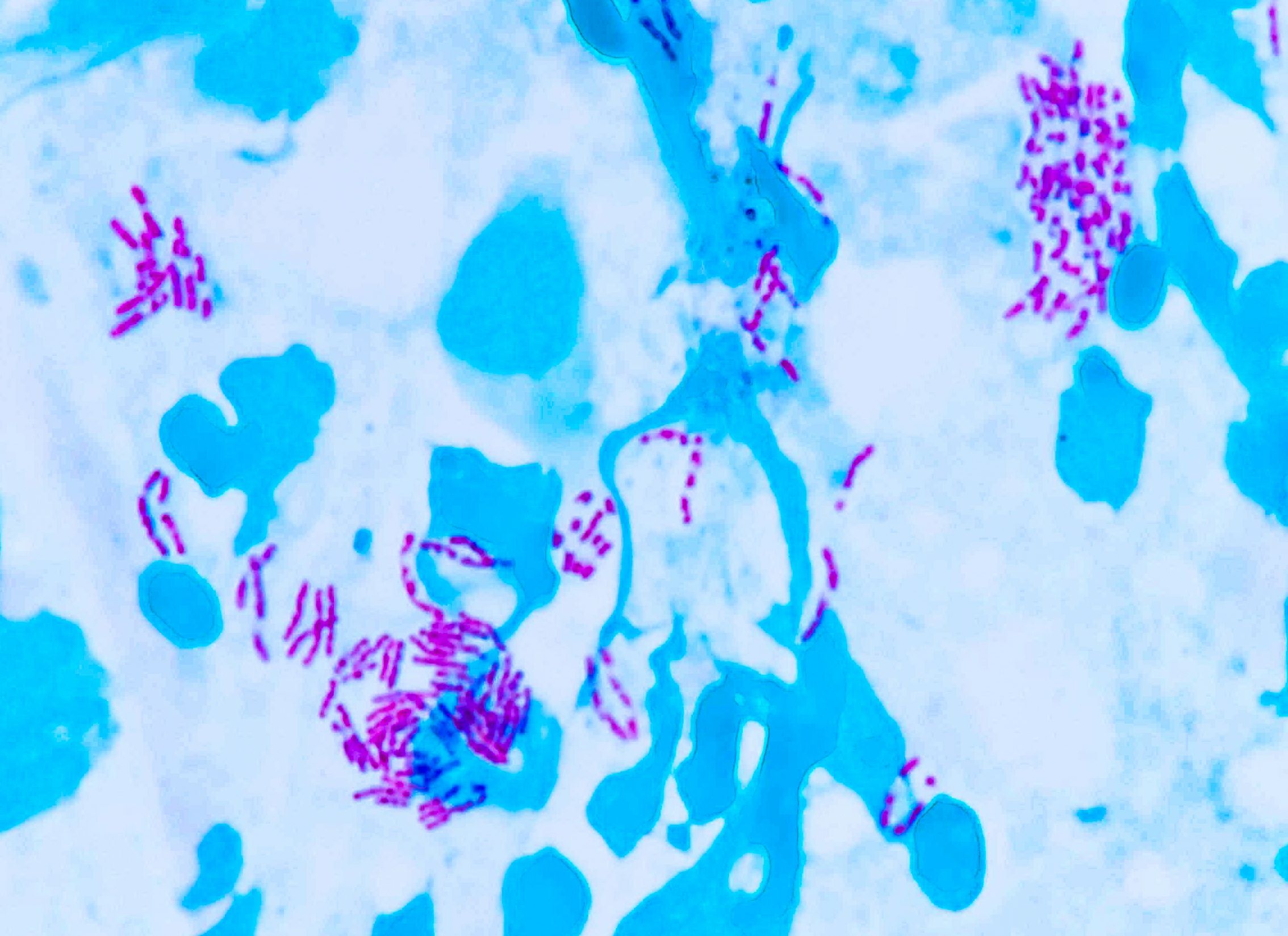
This malachite green counterstain is shown here instead of methylene blue. Both work — just helps you see structure differently.
2. Toxicosis from Transport
Methylene blue isn’t just a dye — it helps detoxify by acting as an electron acceptor/donor.
It can convert methemoglobin back into hemoglobin and reduce oxidative stress in fish.
Use low-dose methylene blue immediately after unpacking — it might save lives.
Untreated toxicosis can lead to kidney necrosis, slow death, and delayed mortality within weeks.
3. Quarantine
If no severe infection is found:
Let the fish rest for 1–3 days. Observe. Then decide.
If anything is suspicious — don’t rush into harsh chemical baths. Many people throw new fish into formalin/malachite green (FMC) straight away. That’s harmful, not helpful. ⚠️ Gentle Rinse, Not a Chemical War
If fish are small and manageable — rinse them manually, in slightly different salinity. Wear gloves.
Marine fish → briefly in freshwater
Freshwater fish → briefly in saline water
No FMC needed unless really justified
Physical slime removal helps more than short chemical exposure.
Long-Term: Keep Watching
In the following days — take new smears, collect feces, and monitor.
Some common threats by water type:
Tropical Saltwater (TS) :
- Skin: Bacteria, Cryptocaryon irritans, Monogeneans (Capsalidae)
- Internal: Cryptobia, Hexamita, Spironucleus, Trichomonas
Tropical Freshwater (TF):
- Skin: Bacteria, fungi, Ichthyophthirius, Ichthyobodo
- Internal: Cryptobia, Hexamita, Spironucleus, Trichomonas, nematodes
Cold Saltwater (CS):
- Skin: Fungi, bacteria — slower development, easier to treat
Cold Freshwater (CF):
- Skin: Trichodina, Chilodonella, Ichthyophthirius, Ichthyobodo, fungi, Gyrodactylus
- Internal: Bacteria, fungi, cestodes
Find them all in the Gallery
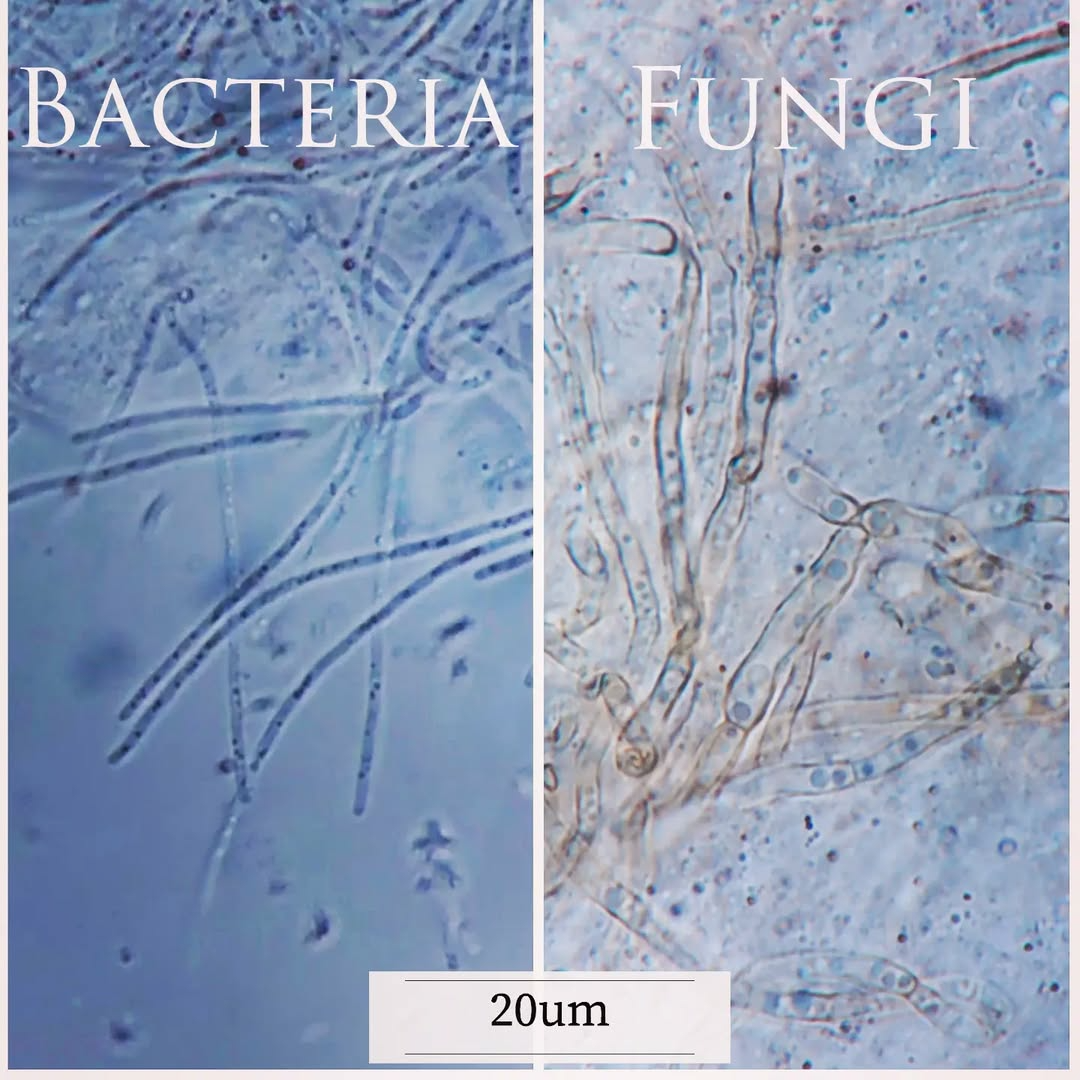
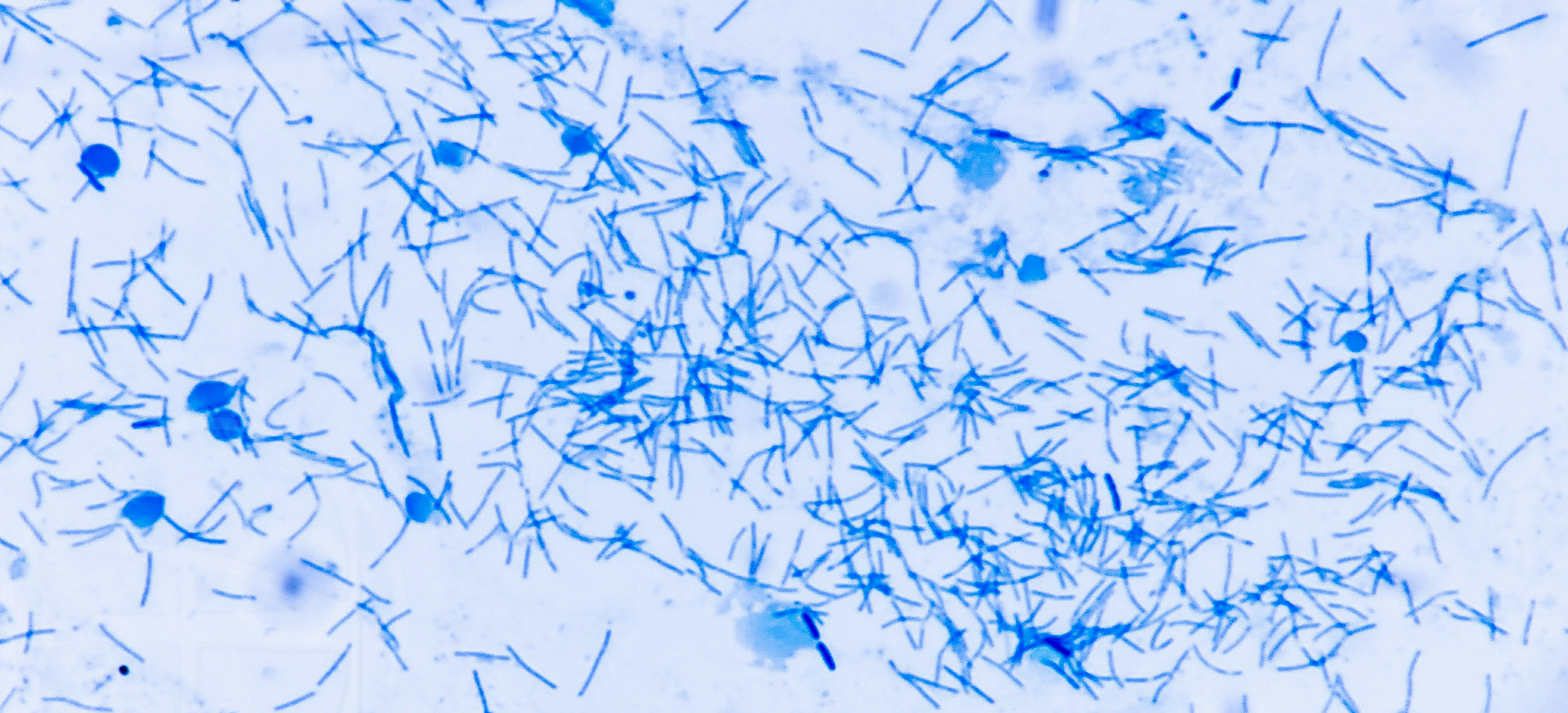
Fungi are usually much larger than bacteria — don’t confuse them in smears!